Research Focus |
|
为什么90%的临床药物开发失败,如何改进? 2024-04-12 17:52:55 浏览次数:995 | |
| 为什么90%的临床药物开发失败,如何改进? 来源:仪方生物 www.yeslab.com 尽管实施了许多成功的策略,但90%的临床药物开发都失败了,这就提出了一个问题,即靶点验证和药物优化的某些方面是否被忽视了?目前的药物优化过度强调使用结构-活性关系(SAR)的效力/特异性,而忽略了使用结构-组织暴露/选择相关性(STR)的疾病/正常组织中的组织暴露/选择性,这可能会误导候选药物的选择,并影响临床剂量/疗效/毒性的平衡。我们提出了结构-组织暴露/选择性-活性关系(STAR)来改进药物优化,该关系根据药物的效力/选择性、组织暴露/选择和平衡临床疗效/毒性所需的剂量对候选药物进行分类。I类药物具有高特异性/效力和高组织暴露/选择性,需要低剂量才能获得卓越的临床疗效/安全性和高成功率。II类药物具有高特异性/效力和低组织暴露/选择性,需要高剂量才能达到高毒性的临床疗效,需要谨慎评估。III类药物的特异性/效力相对较低(足够),但组织暴露/选择性较高,这需要低剂量才能达到具有可控毒性的临床疗效,但往往被忽视。IV类药物特异性/效力低,组织暴露/选择性低,疗效/安全性不足,应尽早终止。STAR可以改善药物优化和临床研究,以获得临床药物开发的成功。 药物发现和开发是一个漫长、昂贵且高风险的过程,需要超过10e15年的时间,每种新药获批用于临床的平均成本超过12亿美元1。对于任何一家制药公司或学术机构来说,在临床前阶段对候选药物进行严格优化后,将候选药物推进I期临床试验都是一项巨大的成就。然而,十分之九的候选药物在进入临床研究后,将在I、II、III期临床试验和药物审批过程中失败2,3。同样值得注意的是,90%的失败率是针对已经进入I期临床试验的候选药物,其中不包括处于临床前阶段的候选药物。如果将临床前阶段的候选药物也计算在内,药物发现/开发的失败率甚至高于90%。 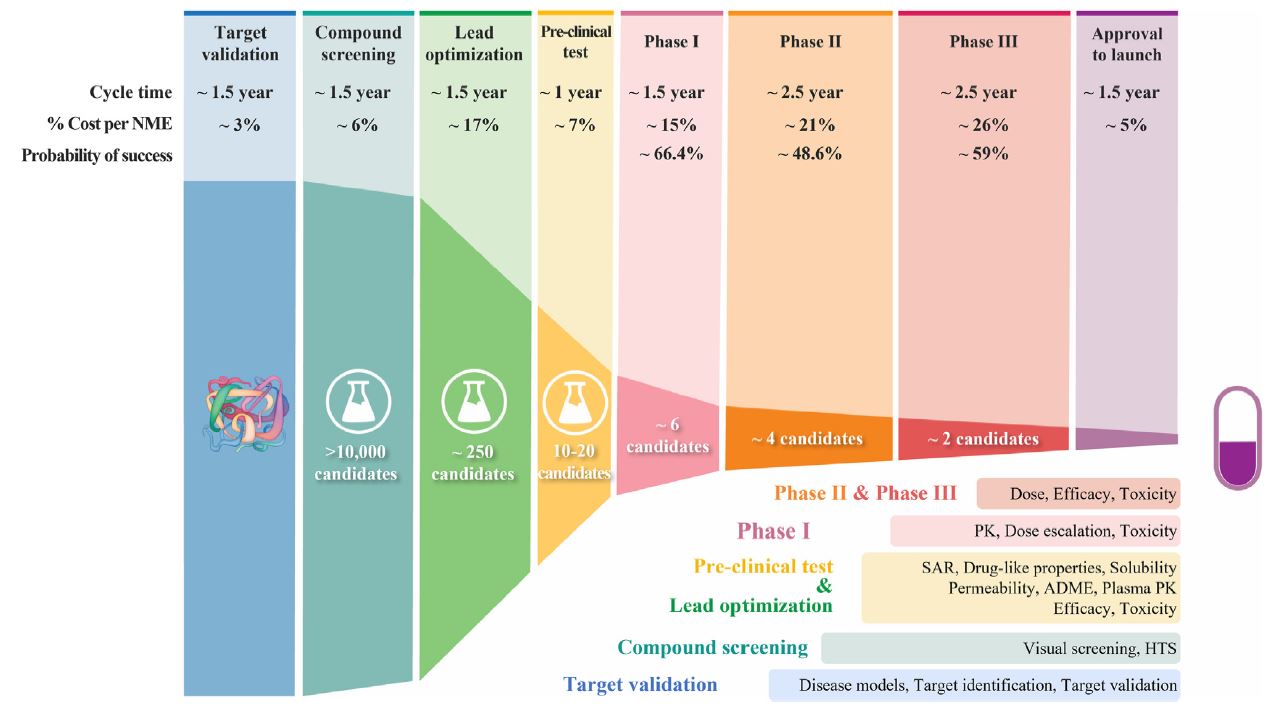 对2010年至2017年临床试验数据的分析显示,90%的药物开发临床失败可能有四个原因:缺乏临床疗效(40%e50%)、无法控制的毒性(30%)、药物样特性差(10%e15%)、缺乏商业需求和战略规划差(10%)2,4。通常,药物开发遵循经典过程(图1),其中包括严格的基因和基因组靶点验证、候选药物分子的高通量筛选(HTS)、严格的药物活性和类药物特性优化、临床前疗效和毒性测试,以及生物标志物引导的患者选择和最佳临床试验设计。在过去的几十年里,上述药物开发的每一步都经过了严格的优化和验证,同时在药物开发过程中正确地实施了许多成功的策略,以选择最佳的候选药物进行临床研究。尽管做出了这些经过验证的努力,但临床药物开发的总体成功率仍然很低,为10%e15%5e7。如此持续的高失败率引发了几个问题:尽管在过去几十年中实施了许多成功的策略,但为什么90%的临床药物开发失败了?我们是否忽视了药物开发过程中导致高度失败的某些方面?如何提高临床药物开发的成功率? 在过去的几十年里,90%的临床药物开发在临床I、II和III期临床研究和药物批准期间失败,原因可能有四个:缺乏临床疗效、无法控制的毒性、不良的类药物特性、缺乏商业需求和糟糕的战略规划。尽管许多成功的策略都得到了正确的实施,以克服90%临床开发失败的四个可能原因,但在过去几十年中,临床药物开发的成功率仍保持在10%e15%。这种高失败率引发了一个问题,即药物开发的某些方面是否被忽视了?一方面,真正的靶点验证,即确认分子靶点是人类疾病的原因和药物的预期靶点,对临床药物开发的成功仍然具有挑战性。另一方面,当前的药物优化可能过分强调了一个方面,而忽略了其他方面,这可能会误导候选药物的选择,并使临床剂量/疗效/毒性失衡。 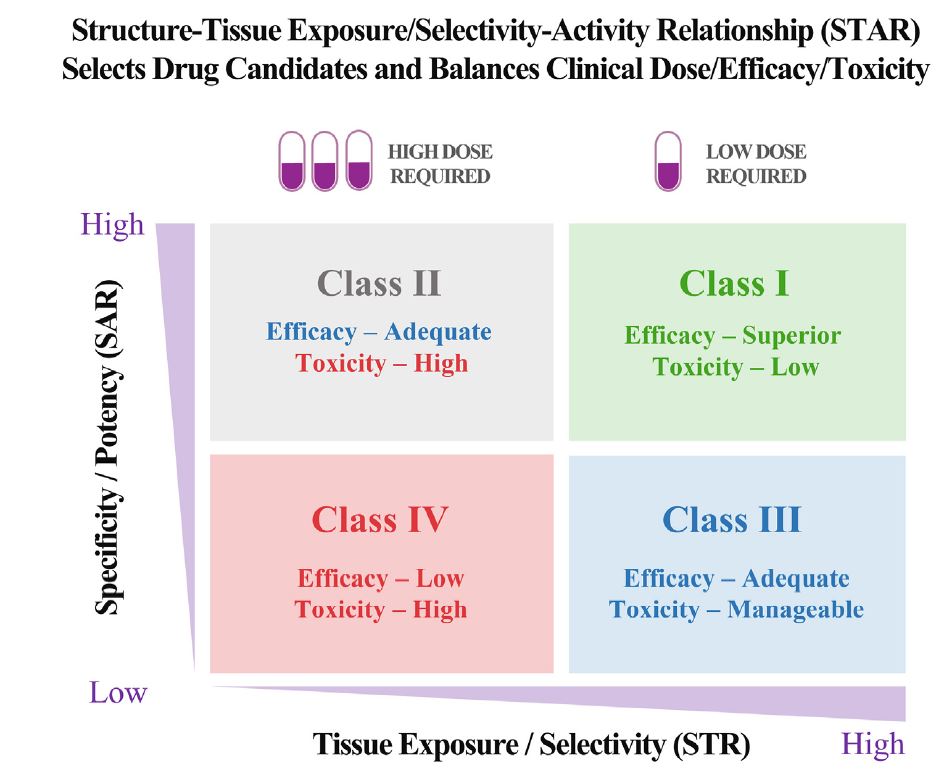 在临床药物开发中,为了在临床剂量、疗效和毒性之间实现微妙的平衡,以优化患者的获益/风险比,理想的候选药物将具有高效力和特异性,以抑制其分子靶标而不产生脱靶效应,在疾病靶向组织中高药物暴露以在最佳剂量(理想情况下为低剂量)下获得足够的疗效,在健康组织中最小药物暴露以避免最佳剂量(即使是高剂量)下的毒性。临床试验中候选药物的临床剂量/疗效/毒性的微妙平衡不仅取决于其抑制其分子靶标的效力/特异性(通过SAR研究),还取决于其在疾病靶向组织和正常组织中的组织暴露/选择性(通过STR研究)。然而,目前的药物优化过程通过SAR研究过度强调了药物的效力/特异性,而通过STR研究忽视了药物在疾病靶向组织与正常组织中的组织暴露/选择性,这可能误导了候选药物的选择,影响了临床剂量优化,并破坏了临床疗效和毒性的平衡。 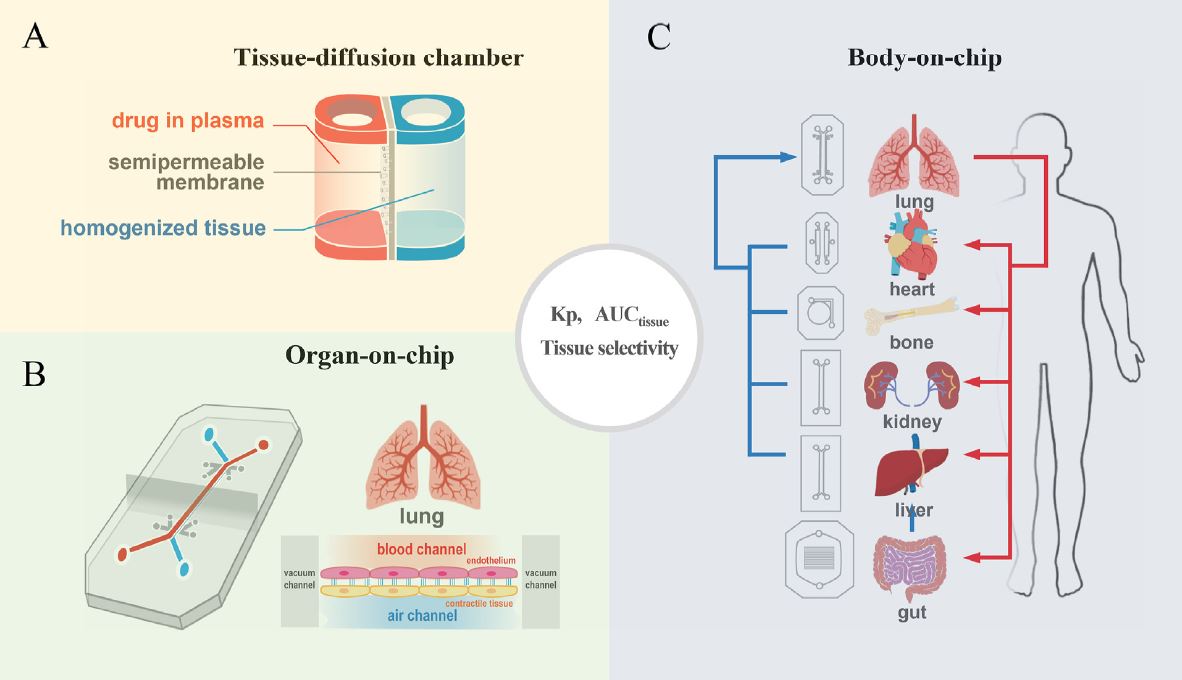 我们提出了一个STAR系统来改进药物优化过程,该系统根据三个方面将候选药物分为四类:药物效力/特异性(高或低)、药物组织暴露/选择性(高或高)和平衡临床疗效/毒性所需的剂量(高或小)。四类不同的候选药物(I-IV类)需要不同的策略来选择主要候选药物,优化临床剂量,并平衡临床疗效/毒性。在该STAR系统中,I类候选药物具有高特异性/效力和高暴露/选择性,这需要低剂量才能实现平衡的疗效/安全性,并且最理想的是具有高成功率。II类候选药物具有高特异性/效力和低组织暴露/选择性,需要高剂量才能达到足够的疗效,但可能具有难以控制的毒性。需要谨慎评估II类候选药物,以平衡临床剂量/疗效/毒性。III类候选药物具有相对较低但足够的特异性/效力,但具有较高的组织暴露/选择性,这可能需要低至中等剂量才能获得足够的疗效和可控的毒性。III类候选药物可能具有很高的临床成功率,但由于在药物发现的早期阶段血浆药物暴露不佳,因此经常被忽视。IV类候选药物具有低特异性/效力和低暴露/选择性,这通常需要高剂量,并且表现出不充分的疗效和高毒性,应尽早终止。未来,STAR系统可以使用人工智能辅助计算建模、体外筛选、体内测试和无创成像技术进行改进。STAR的应用将提高四类不同候选药物的药物优化和临床研究效率,提高临床药物开发的成功率。 Why 90% of clinical drug development fails and how to improve it? Ninety percent of clinical drug development fails despite implementation of many successful strategies, which raised the question whether certain aspects in target validation and drug optimization are overlooked? Current drug optimization overly emphasizes potency/specificity using structure‒activityrelationship (SAR) but overlooks tissue exposure/selectivity in disease/normal tissues using structure‒tissue exposure/selectivityerelationship (STR), which may mislead the drug candidate selection and impact the balance of clinical dose/efficacy/toxicity. We propose structure‒tissue exposure/selectivityeactivity relationship (STAR) to improve drug optimization, which classifies drug candidates based on drug’s potency/ selectivity, tissue exposure/selectivity, and required dose for balancing clinical efficacy/toxicity. Class I drugs have high specificity/potency and high tissue exposure/selectivity, which needs low dose to achieve superior clinical efficacy/safety with high success rate. Class II drugs have high specificity/potency and low tissue exposure/selectivity, which requires high dose to achieve clinical efficacy with high toxicity and needs to be cautiously evaluated. Class III drugs have relatively low (adequate) specificity/ potency but high tissue exposure/selectivity, which requires low dose to achieve clinical efficacy with manageable toxicity but are often overlooked. Class IV drugs have low specificity/potency and low tissue exposure/selectivity, which achieves inadequate efficacy/safety, and should be terminated early. STAR may improve drug optimization and clinical studies for the success of clinical drug development. Drug discovery and development is a long, costly, and high-risk process that takes over 10e15 years with an average cost of over $1e2 billion for each new drug to be approved for clinical use1. For any pharmaceutical company or academic institution, it is a big achievement to advance a drug candidate to phase I clinical trial after drug candidates are rigorously optimized at preclinical stage. However, nine out of ten drug candidates after they have entered clinical studies would fail during phase I, II, III clinical trials and drug approval2,3. It is also worth noting that the 90% failure rate is for the drug candidates that are already advanced to phase I clinical trial, which does not include the drug candidates in the preclinical stages. If drug candidates in the preclinical stage are also counted, the failure rate of drug discovery/development is even higher than 90%. Analyses of clinical trial data from 2010 to 2017 show four possible reasons attributed to the 90% clinical failures of drug development: lack of clinical efficacy (40%e50%), unmanageable toxicity (30%), poor drug-like properties (10%e15%), and lack of commercial needs and poor strategic planning (10%)2,4. Generally, drug development follows a classical process (Fig. 1), which includes vigorous genetic and genomic target validation, highthroughput screening (HTS) of drug candidate molecules, rigorously drug optimization for activity and drug-like properties, preclinical efficacy and toxicity testing, and biomarker-guided selection of patients and optimal clinical trial designs. In the past few decades, each step of the above drug development has been rigorously optimized and validated, while many successful strategies have been rightfully implemented in the drug development process to select the best drug candidates for clinical studies. Despite this validated effort, the overall success rate of clinical drug development remains low at 10%e15%5e7. Such persistent high failure rate raises several questions: Why 90% of clinical drug development fails despite implementation of many successful strategies in the past several decades? Did we overlook certain aspects of drug development process leading to high failure? How can we improve the success rate of clinical drug development? In the past few decades, 90% of clinical drug developments have failed during clinical phase I, II, and III clinical studies and drug approval due to four possible reasons: lack of clinical efficacy, unmanageable toxicity, poor drug-like properties, and lack of commercial needs and poor strategic planning. Although many successful strategies are correctly implemented to overcome the four possible reasons of 90% of clinical development failures, the success rate of clinical drug development remains at 10%e15% in the past few decades. This high failure rate raises the question of whether certain aspects of drug development are overlooked? On the one hand, true target validation, which confirms the molecular target is the cause of human disease and drug’s intended target, is still challenging for the success of clinical drug development. On the other hand, current drug optimization may have overemphasized one aspect but overlooked others that may mislead drug candidate selection and unbalance clinical dose/efficacy/ toxicity. In clinical drug development, in order to achieve a delicate balance among clinical dose, efficacy, and toxicity to optimize the benefit/risk ratios in patients, an ideal drug candidate would have high potency and specificity to inhibit its molecular target without off-target effect, high drug exposure in disease-targeted tissues to achieve adequate efficacy at an optimal dose (ideally at low doses), and minimal drug exposure in healthy tissues to avoid toxicity at optimal doses (even at high doses). The delicate balance of clinical dose/efficacy/toxicity of drug candidates in clinical trials is not only determined by their potency/specificity in inhibiting its molecular targets (through SAR studies), but also by their tissue exposure/selectivity in disease-targeted tissues and normal tissues (through STR studies). However, current drug optimization process overly emphasized drug’s potency/specificity through SAR studies but overlooked drug’s tissue exposure/ selectivity in disease-targeted tissues vs. normal tissues through STR studies, which may have misled the drug candidate selection, impacted clinical dose optimization, and tipped the balance of clinical efficacy and toxicity. We propose a STAR system to improve the drug optimization process, which classifies drug candidates into four different classes based on three aspects: drug potency/specificity (high or low), drug tissue exposure/selectivity (high or low) and required dose for balancing clinical efficacy/toxicity (high or low). The four different classes of drug candidates (classes I‒IV) require different strategies to select lead drug candidates, optimize clinical doses, and balance clinical efficacy/toxicity. In this STAR system, class I drug candidates have high specificity/potency and high exposure/ selectivity, which requires low dose to achieve balanced efficacy/ safety and are most desirable with a high success rate. Class II drug candidates have high specificity/potency and low tissue exposure/selectivity, which needs a high dose to achieve adequate efficacy but may have unmanageable toxicity. Class II drug candidates need to be cautiously evaluated to balance clinical dose/ efficacy/toxicity. Class III drug candidates have relatively low but adequate specificity/potency but high tissue exposure/selectivity, which may require a low to medium dose to achieve adequate efficacy with manageable toxicity. The class III drug candidates may have a high clinical success rate but are often overlooked due to poor plasma drug exposure at an early stage of drug discovery. Class IV drug candidates have low specificity/potency and low exposure/selectivity, which often requires high dose and shows inadequate efficacy with high toxicity and should be terminated as early as possible. In the future, the STAR system can be improved using AI-aided computation modeling, in vitro screening, in vivo testing, and non-invasive imaging technology. Application of STAR will improve the efficiency of drug optimization and clinical studies for four different classes of drug candidates to improve the success rate of clinical drug development. |