Research Focus |
|
反义寡核苷酸治疗Timothy综合征 2024-04-29 20:59:30 浏览次数:2476 | |
| Timothy综合征(TS)是一种严重的多系统疾病,以自闭症、癫痫、长QT综合征和其他神经精神疾病为特征1。TS 1型(TS1)是由选择性剪接和发育富集的CACNA1C外显子8A中的功能获得变体引起的,而不是其对应的外显子8。我们之前在TS1患者的神经元中发现了几种表型,包括通道失活延迟、去极化诱导的钙升高延长、神经元间迁移受损、活性依赖性树突回缩和外显子8A2-6的意外持续表达。我们推断,将CACNA1C外显子利用率从8A转换为8将代表一种潜在的治疗策略。在这里,我们开发了反义寡核苷酸(ASOs),以在体外和体内移植后有效减少人类细胞中外显子8A的包含。我们发现ASO介导的从第8A外显子到第8外显子的转换有力地挽救了患者来源的皮质类器官的缺陷和前脑组装体的迁移。利用先前开发的移植平台7,我们发现单次鞘内ASO给药挽救了患者神经元的钙变化和体内树突回缩,这表明抑制CACNA1C外显子8A表达是TS1的潜在治疗方法。从广义上讲,这些实验说明了基于多水平、体内和体外干细胞模型的方法如何确定逆转疾病相关神经病理生理学的策略。 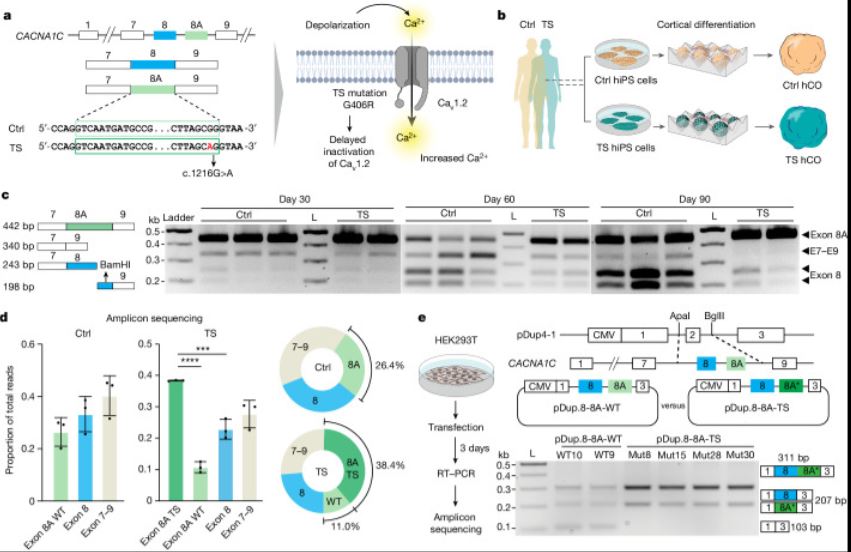 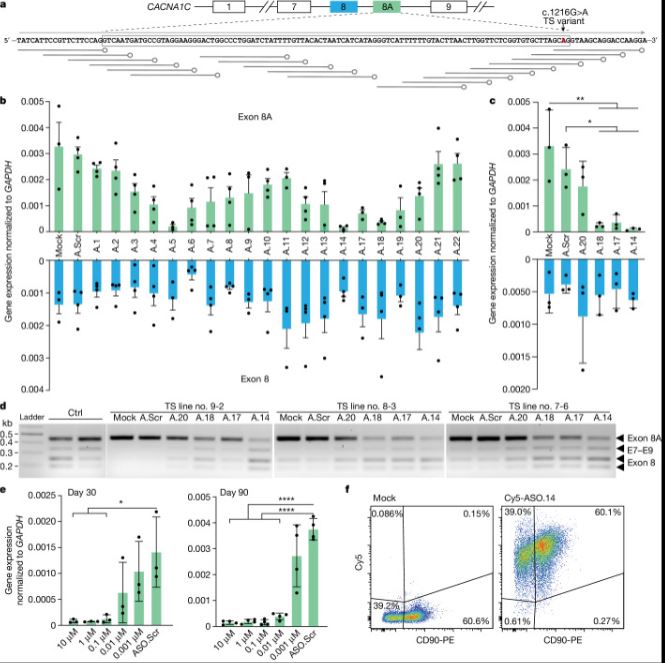 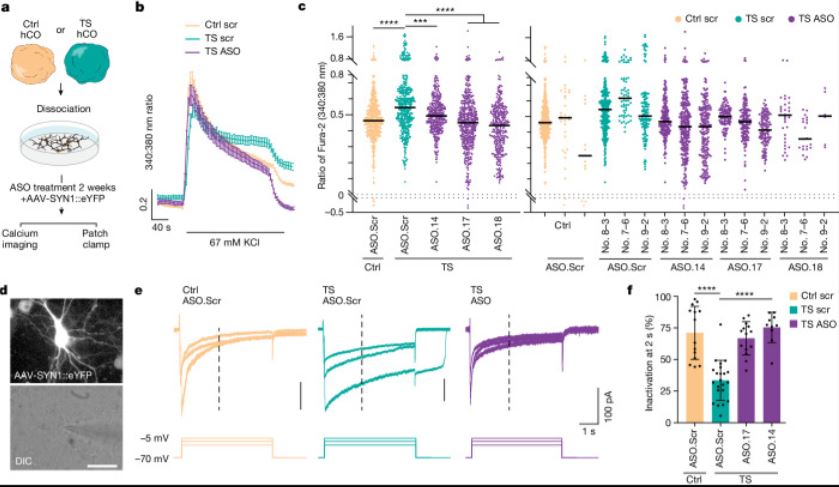 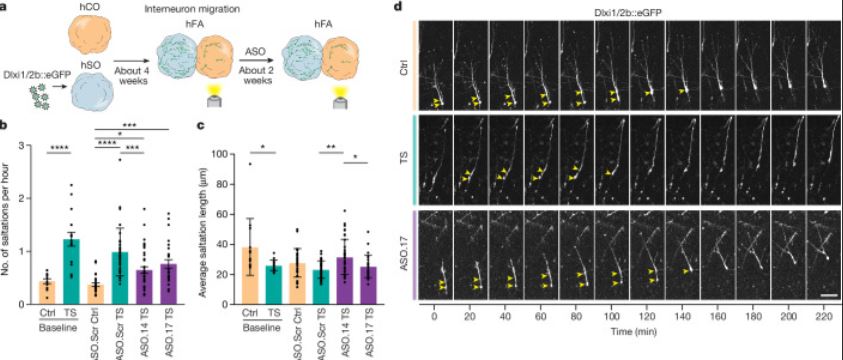 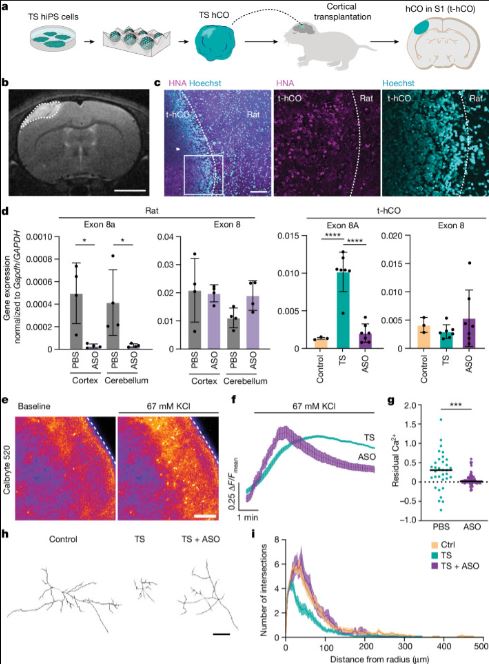 Antisense oligonucleotide therapeutic approach for Timothy syndrome Timothy syndrome (TS) is a severe, multisystem disorder characterized by autism, epilepsy, long-QT syndrome and other neuropsychiatric conditions1. TS type 1 (TS1) is caused by a gain-of-function variant in the alternatively spliced and developmentally enriched CACNA1C exon 8A, as opposed to its counterpart exon 8. We previously uncovered several phenotypes in neurons derived from patients with TS1, including delayed channel inactivation, prolonged depolarization-induced calcium rise, impaired interneuron migration, activity-dependent dendrite retraction and an unanticipated persistent expression of exon 8A2-6. We reasoned that switching CACNA1C exon utilization from 8A to 8 would represent a potential therapeutic strategy. Here we developed antisense oligonucleotides (ASOs) to effectively decrease the inclusion of exon 8A in human cells both in vitro and, following transplantation, in vivo. We discovered that the ASO-mediated switch from exon 8A to 8 robustly rescued defects in patient-derived cortical organoids and migration in forebrain assembloids. Leveraging a transplantation platform previously developed7, we found that a single intrathecal ASO administration rescued calcium changes and in vivo dendrite retraction of patient neurons, suggesting that suppression of CACNA1C exon 8A expression is a potential treatment for TS1. Broadly, these experiments illustrate how a multilevel, in vivo and in vitro stem cell model-based approach can identify strategies to reverse disease-relevant neural pathophysiology. |
